Overview
We are inspired by Nature, which utilizes the abundant first row transition metal elements as co-factors in metalloenzymes to catalyze the most difficult of regio- and stereo-selective chemical reactions. Biology does this more cleanly and efficiently that the state of the art in synthetic chemistry. The cornerstone of these processes is the “activation” of O2, N2, H2O and CO2. These small, abundant, stable, non-toxic, (gaseous) molecules are the source of C, H, N and O building blocks for the biosynthesis of essential biomolecules like proteins, DNA and hormones. Metalloenzymes provide blueprints for the new catalysts we need to discover for managing chemical feedstocks and sustainable ensuring element recycling. This is quite simple the answer for mitigating the Greenhouse effect, reducing chemical waste and finding new technologies for solar powered energy generation.
Coordination Chemistry: By tailoring organic ligand scaffolds we aim to construct functional supramolecular assemblies which can mimic proteins and control the primary and secondary coordination spheres of transition metal ions. We create active sites which promote the transformation of O2, H2O and CO2 in similar ways to the metalloenzymes.
Research training: Students in the group become all-rounders. They gain experience in organic, organometallic and inorganic synthesis methodologies, a wide range of spectroscopic (NMR, EPR, UV-vis, IR, Raman, VCD Mössabuer, EXAFS), mass spectrometric (ESI, MALDI), electrochemical and X-ray diffraction (single crystal and powder) and GC, HPLC techniques for characterization of new materials and catalytic reactions.
Green chemistry: Better ways to catalyze oxidation reactions
Oxidation reactions in pharmaceutical manufacturing are highly inefficient compared to their biochemical counterparts where atom efficient catalysis by Fe, Cu, Mn metalloenzymes takes the center stage in the biosynthesis of organic molecules. It has been calculated that one tonne of chemical waste is produced for every one kg of a active pharmaceutical ingredient. We need to develop similarly efficient, cheap and sustainable regio- and stereoselective oxidation catalysis for chemical synthesis and efficient atom recycling. Molecular iron model complexes which mimic the bioreactivity of non-heme iron enzymes are one solution for catalyst design. Our new iron catalyst shows a structure, and flexibility, more closely related to the microenvironment of the active sites of non-heme metalloenzymes. The choice of terminal oxidant (e.g. O2, H2O2, ROOH, hypochlorite, hypervalent iodine reagents) and the solvent medium, guides the catalyst on either a selective or promiscuous oxidation pathway. This is a remarkable diversity in reactivity mirrors the impressive scope of the non-heme metalloenzymes. Mechanistic studies of our catalysts using spectroscopic analysis of short-lived iron-based oxidants allows for an evolution of structure-activity relationships in the key steps of substrate oxidations.
The recent example below shows cooperative co-activation of water and hypochlorite by a dinuclear (non-heme) diiron(III) complex (McPherson et al. J. Am. Chem. Soc., 2021, 143, 15400–15412).
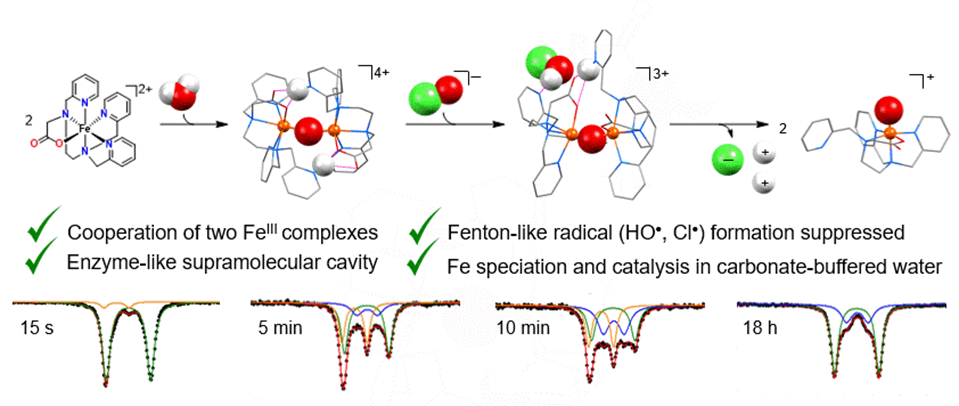
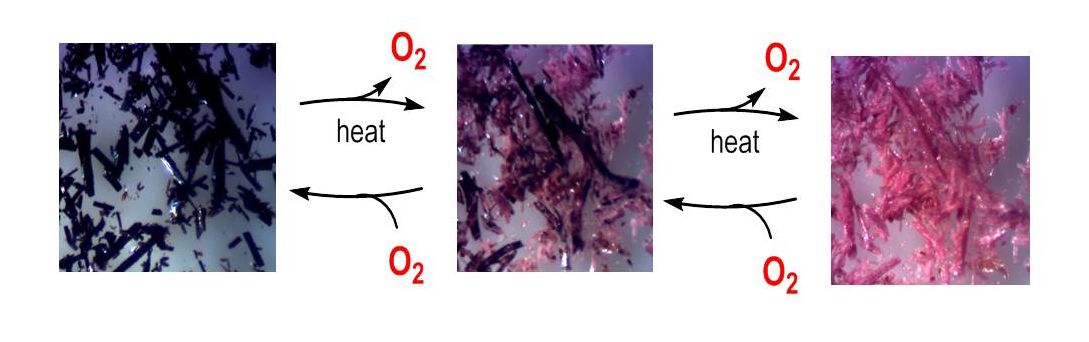
In-crystal gas separation and processing
Crystalline molecular compounds are often mistakenly perceived as rigid and inflexible, in fact small guest molecules can
migrate rapidly through supramolecular crystal lattices. Akin to metalloenzymes, the metal-organic materials we design and
synthesize for gas uptake and processing are chemisorptive. This means the gas chemically bonds to active sites. These new
materials could be applied to the separation and concentration of O2 from air. Rapid transport due to cooperativity between
the dynamic guest gas and flexible host crystal is important for the O2 binding and release mechanisms in reversible gas-solid
reactions which occur in single crystal-to-single crystal transformations.
Selective recognition and binding of O2 to a metal ion is just an electron push away from its activation. We therefore
believe that we will eventually be able to tune the O2 binding sites for the purpose of using O2 directly from air as a
benign terminal oxidant for the oxidation of another guest substrate inside a crystalline lattice.
Electrocatalysis
We have applied our catalysts to the electrocatalytic oxidation of organic compounds. Remarkably this reaction can be done in water. Our next step is to immobilize these systems for the implementation of this discovery to water remediation. In recent years trace pesticides has been an increasing problem in Denmark and this new technology may effect detoxification, or in the best case scenario, the total mineralization of water pollutants.


Artificial photosynthesis (AP)
Electro- and photocatalysis at the interface of synthetic chemistry, materials, nanoscience, and engineering is needed for the creation of chemical energy (fuel) from sunlight. We need to understand how to tune the redox potentials of base metal complexes which can activate the key small molecules, H2O, O2, H2 and CO2, and learn how light can be used to perform chemistry.
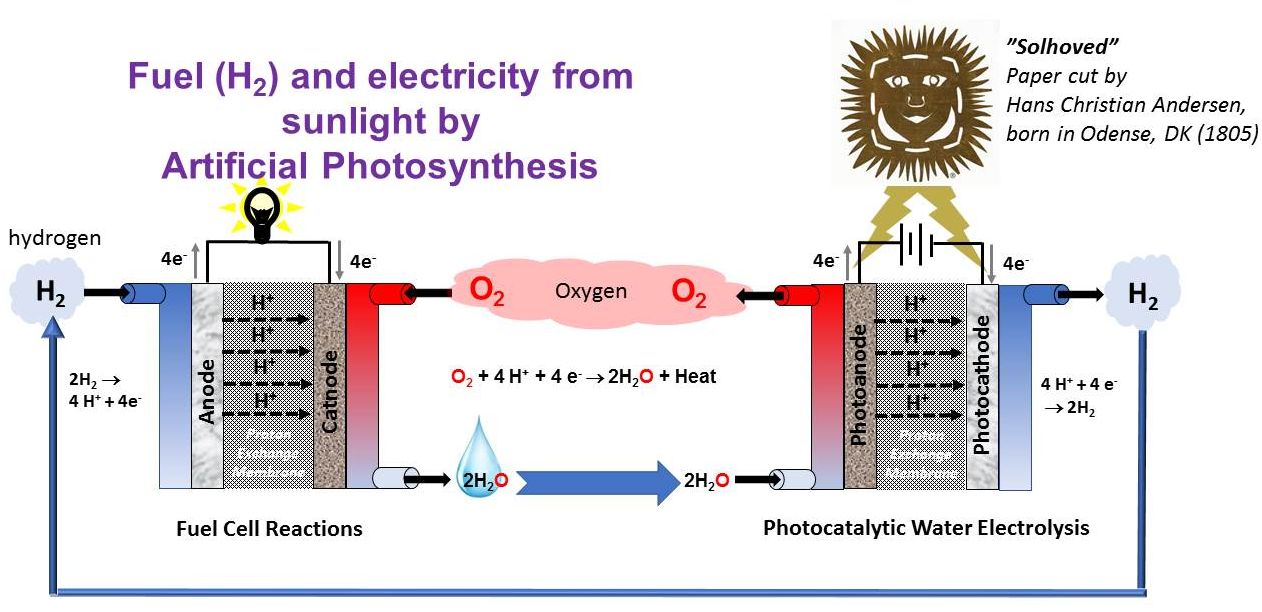
The missing links for realizing AP are the catalysts for photocatalytic water splitting and CO2 reduction (to ultimately produce energy rich molecules like H2, H2CO and H3COOH). We have discovered key catalytic water oxidation by Mn complexes and Fe- and Co- catalyzed O2 reduction reactions. These are key discoveries on the path to realization of AP.
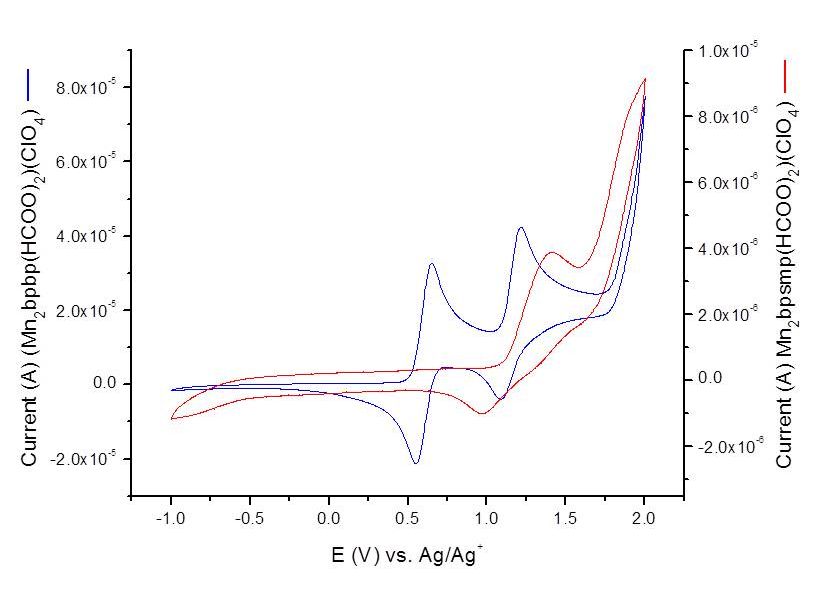
Ligand Tuning of metal redox potentials
Dalton Trans, 2011, 40, 3336 - 3345.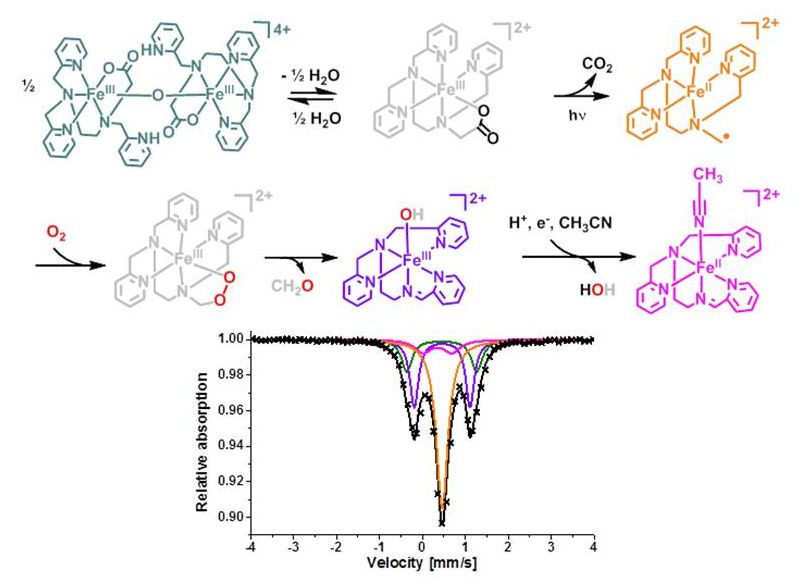
Photoinduced O2-dependent Stepwise Oxidative Deglycination
J. Am. Chem. Soc., 2018, 140, 14150–14160 .
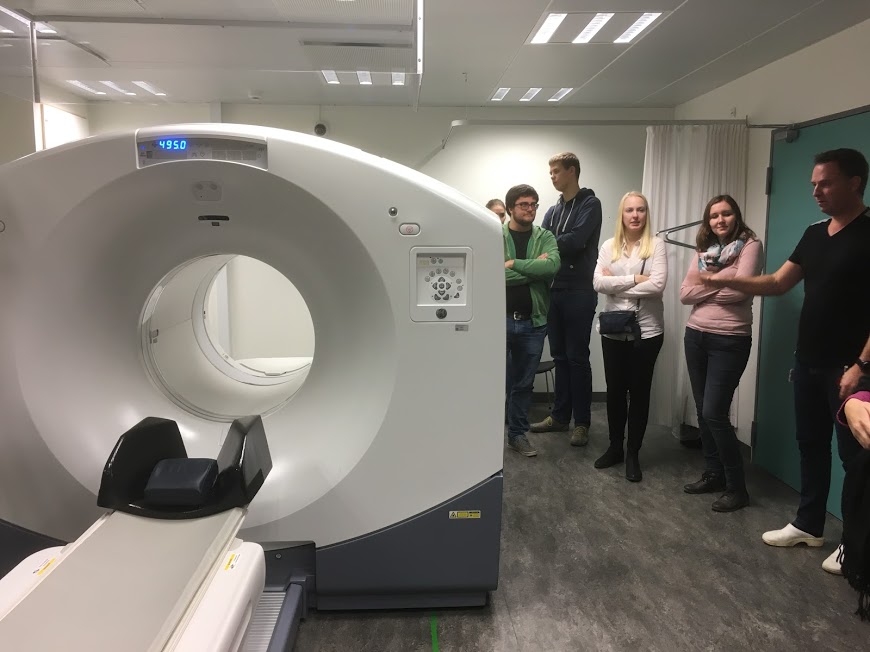
Medicinal Inorganic Chemistry - Radiopharmaceuticals
In collaboration with physicists at the Odense University Hospital we aim to prepare ligands that will be suitable to use
as vectors for implementing as yet unused radioisotopes in nuclear medicine. Potentially different isotopes of the same
element can be applied to imaging and treatment respectively. This means that the same metal coordination complex could be
used for both diagnostic imaging and cancer cell killing therapy. What the clinic lacks to achieve this is new ligands
which we can design and make.
The photo shows an excursion to the Odense University Hospital PET center as part of KE810.
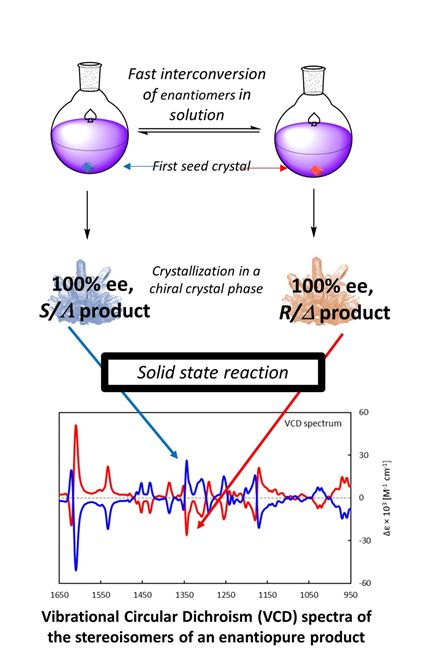
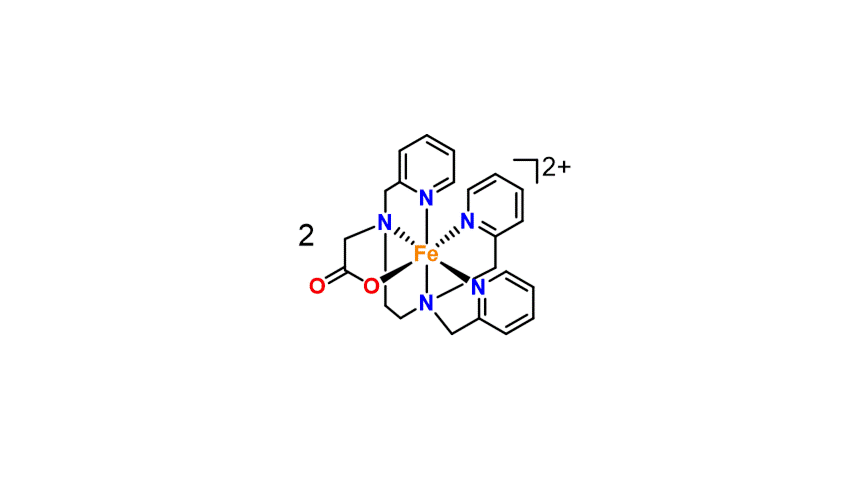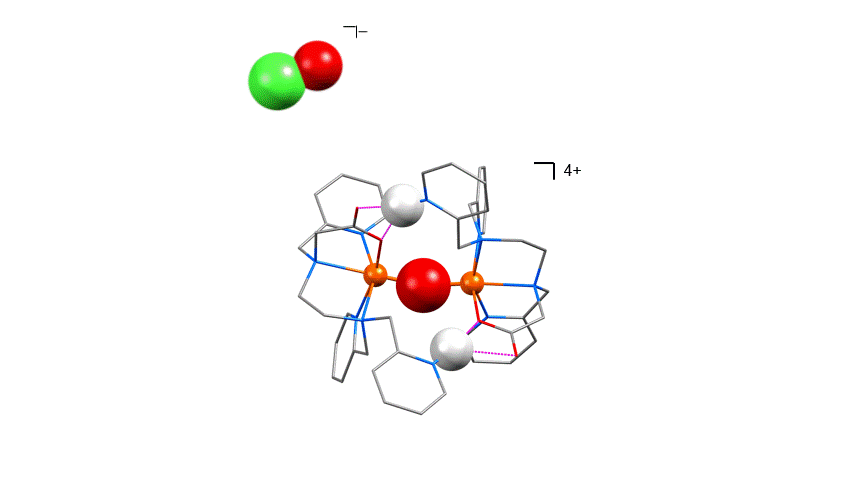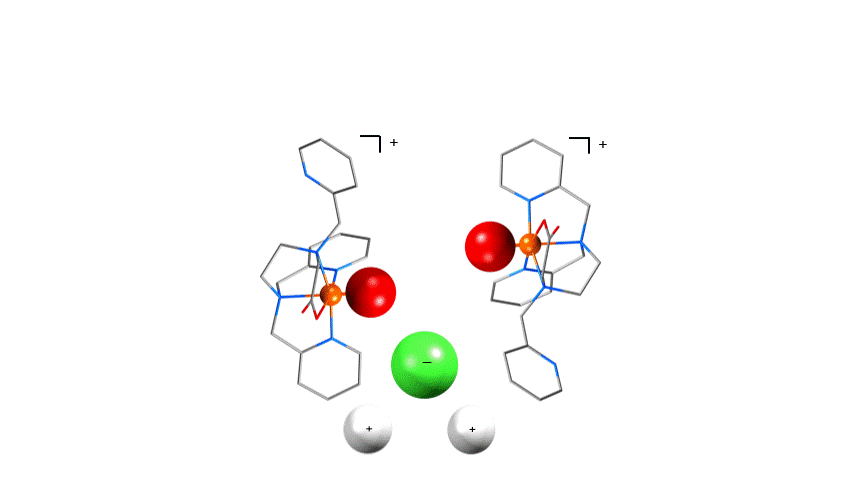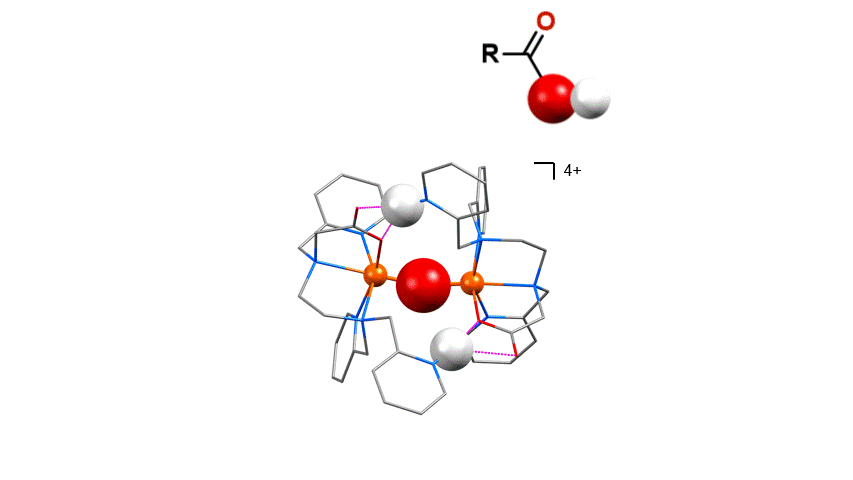
Absolute Asymmetric synthesis
Chirality is an inherent property of biology. A consequence is that many pharmaceuticals show stereochemistry-dependent
pharmokinetics. Less recognized, and certainly not yet utilized, is the fact that crystal phases can also be inherently
chiral – even if the molecular content is achiral.
We hope to put this property to synthetic use by using achiral reagents trapped in chiral crystalline phases in solid state
syntheses (left). By doing this both enantiomers of a desired chiral compound will be equally available.
Chemical Biology
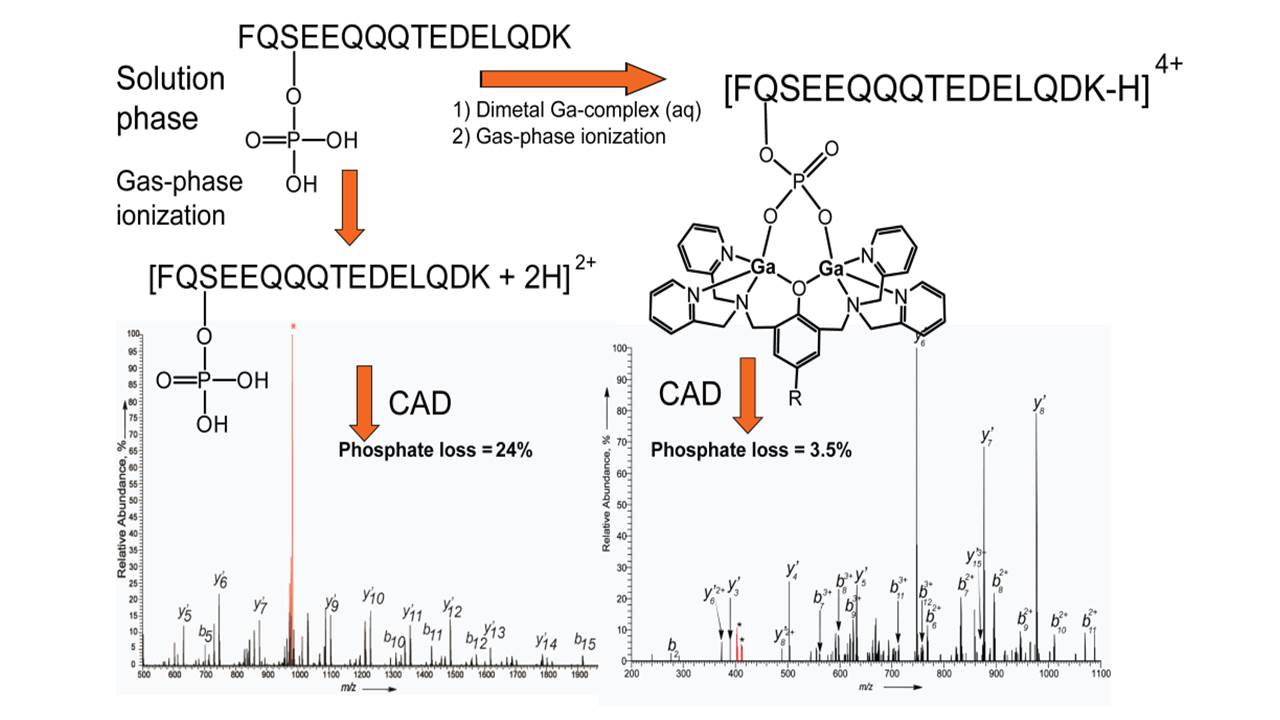
We have designed compounds that can specifically recognize biological motifs. For example the phosphate esters on
post-translationally phosphorylated peptides. The compounds stick to the peptide at the point of modification and this
point can then be recognized using mass spectrometry.
Right: Proteomics - Dimetal recognition of peptide phosphate ester groups (Angew. Chemie, Int. Ed. 2012, 51, 3216-3219)

 Prof Christine McKenzie
Prof Christine McKenzie