Cytochrome c.
This page shows some interactive JSmol views of Cytochrome c, a heme electron transfer metalloprotein.
This structure may be a little slower to load because it is taken from NMR data.
In the inital, conventional, view the Fe(III) form of the protein is shown in cartoon form [Cyt c (oxidised)].
It is a very small, globular protein.
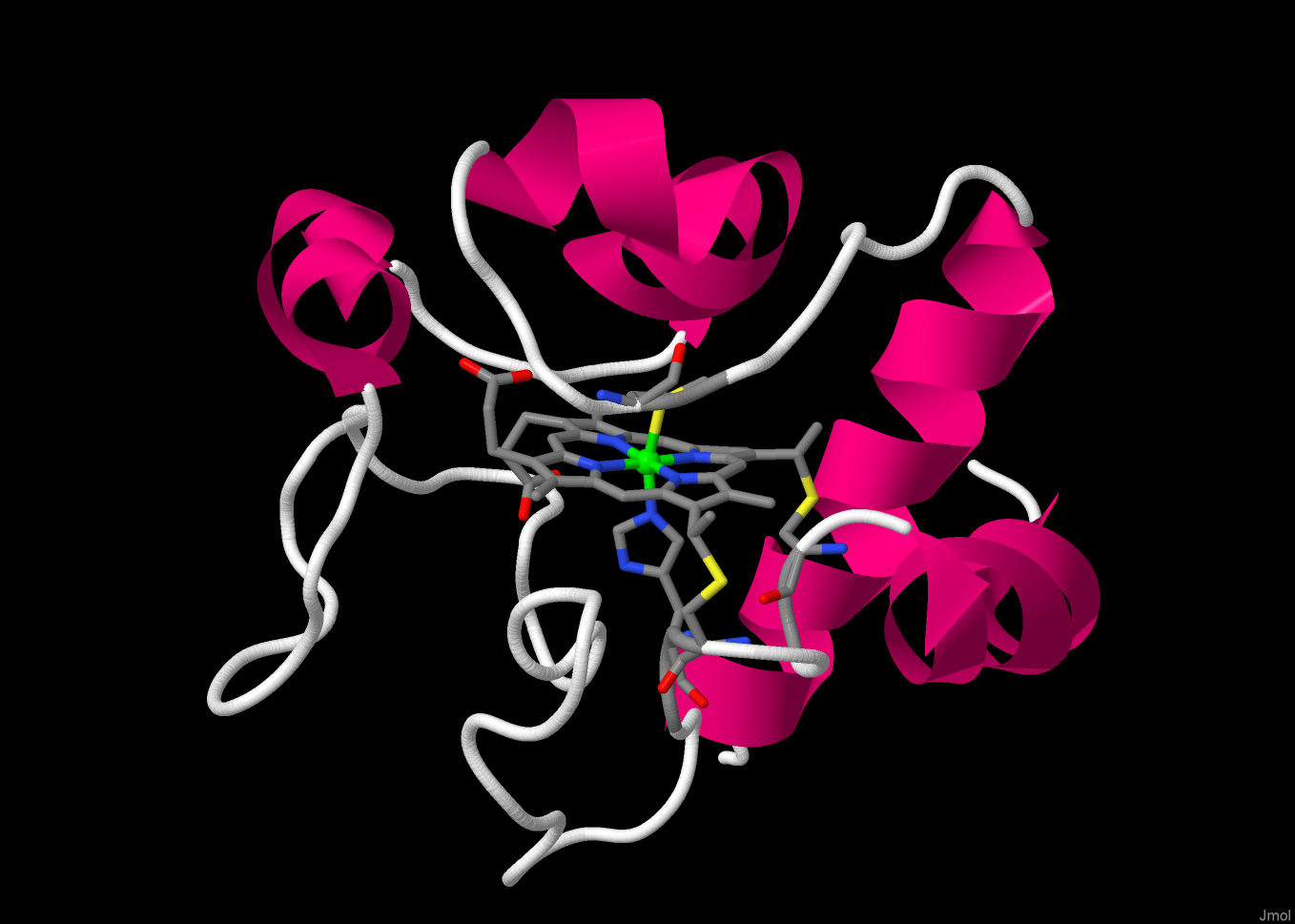
The heme group is attached to the protein via covalent links to two Cys groups - and by two coordinate bonds to the metal ion.
There is little obvious difference between the oxidised and reduced form, [Cyt c (oxidised)], as expected for redox
metalloproteins). For clarity the diagrams show iron(III) as orange and iron(II) as bright green.
[active site] focuses in on the heme group.
The metal ion is 6-coordinate, bound to the four N-donors of the porphyrin group and to axial amino acid residies,
one His and one Met. Note that the two Cys residues bonding to the heme and the coordinating His are from the same short
section of the protein (Cys14 - Ser15 - Gln16 - Cys-17 - His18). The second axial ligand, Met80, is from a different
part of the protein chain.
The edge of the heme group is exposed to solvent.
This is the likely route by which electrons enter and leave.
The final figure shows cytochrome c3, a cytochrome containing four heme groups bound
within a protein approx the same size as cytochrome c. Compare the extent of the solvent exposure
of the hemes in the two cytochromes.
Return to the index page.
Return to the index page.