Iron Sulfur Proteins.
This page shows some interactive JSmol views of iron sulfur proteins. There are several classes of iron-sulfur proteins (below) all of these are involved in electron transfer processes.
Rubredoxin is a small redox metalloprotein, containing one iron ion bound to four Cys residues.
The geometry at the iron is approximately tetrahedral and the metal ion cycles between +3 and +2 states.
Note that the iron is close to the edge of the protein (why?).
Also note the similarity to the type 1 copper site.
The Aquifex aeolicus protein contains one [2Fe-2S] site in which the two iron ions are linked by two inorganic sulfide ion (S2-) bridges. Each iron also has two cysteine ligands and the cluster could be described as comprising two edge-sharing tetrahedra. Note the similarities and differences to the rubredoxin site (above).
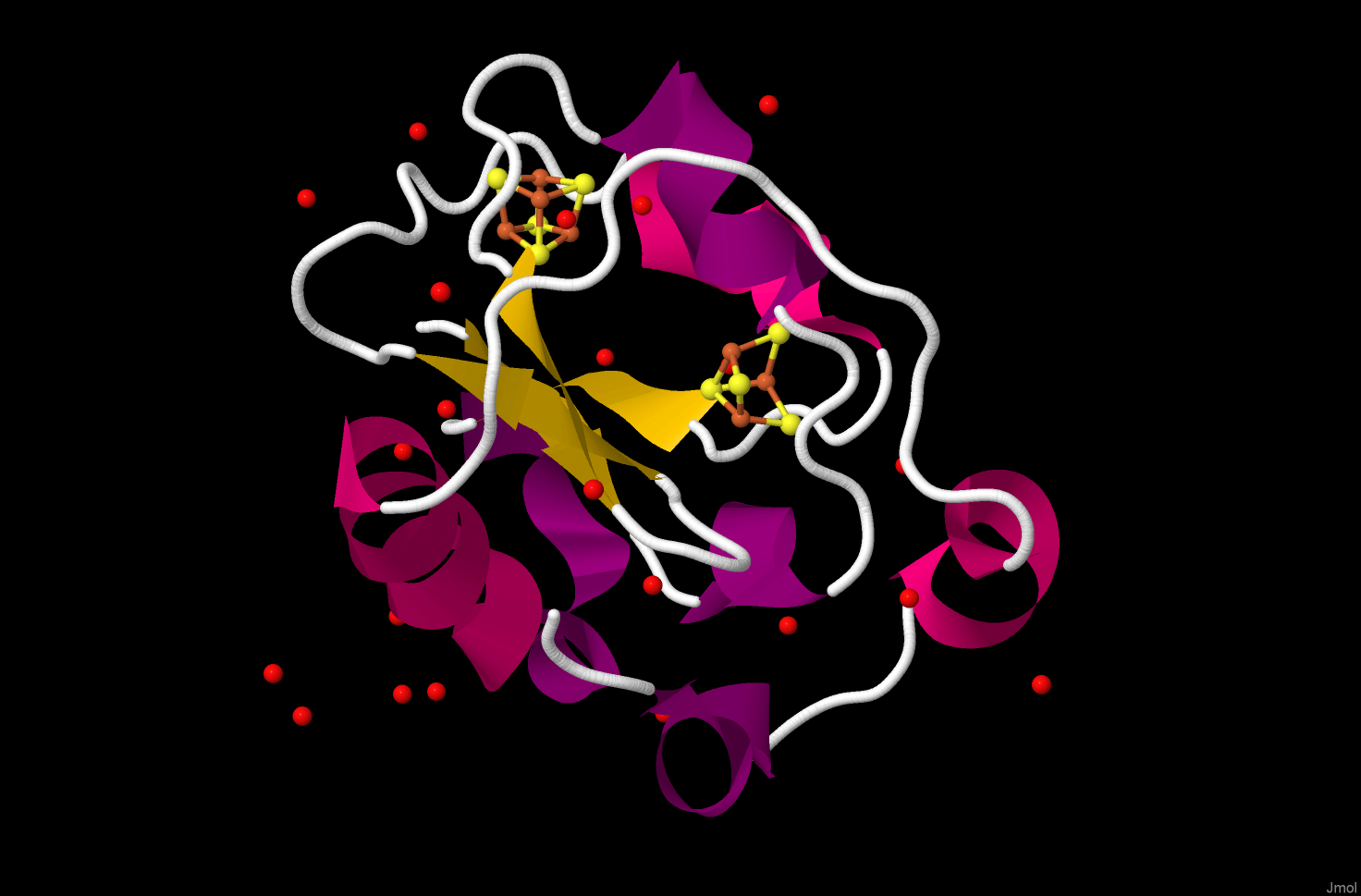
The Azobacter metalloprotein contains two different iron sulfur clusters, one [4Fe-4S] and one, less common
[3Fe-4S] site.
The geometry at the iron ions is still approximately tetrahedral, and that both of these clusters contain four
sulfide ions. In each case the iron ions have three sulfide ligands and one cysteine.
The [3Fe-4S] cluster can be described as an incomplete cubane or as three linked FeS4 tetrahedra.
Synthetic [4Fe-4S] clusters are not difficult to symthesise and a number of model complexes have been prepared, one is shown
under the [model] button.
Return to the index page.
[2Fe-2S] data from: High-resolution crystal structures of the wild type and Cys-55-->Ser and Cys-59-->Ser variants of the thioredoxin-like [2Fe-2S] ferredoxin from Aquifex aeolicus. Yeh, A.P., Ambroggio, X.I., Andrade, S.L.A., Einsle, O., Chatelet, C., Meyer, J., Rees, D.C. (2002) J.Biol.Chem. 277: 34499-34507.
[4Fe-4S] and [3Fe-4S] data from: Azotobacter vinelandii ferredoxin I. Aspartate 15 facilitates proton transfer to the reduced [3Fe-4S] cluster. Shen, B., Martin, L.L., Butt, J.N., Armstrong, F.A., Stout, C.D., Jensen, G.M., Stephens, P.J., La Mar, G.N., Gorst, C.M., Burgess, B.K. (1993) J.Biol.Chem. 268: 25928-25939.
Model data from: Stephan, D.W., Papaefthymiou, G.C., Frankel, R.B., Holm, R.H. (1983) Inorg. Chem. 22, 1550.
Return to the index page.