Zinc Fingers.
This page shows some interactive JSmol views of a zinc finger protein.
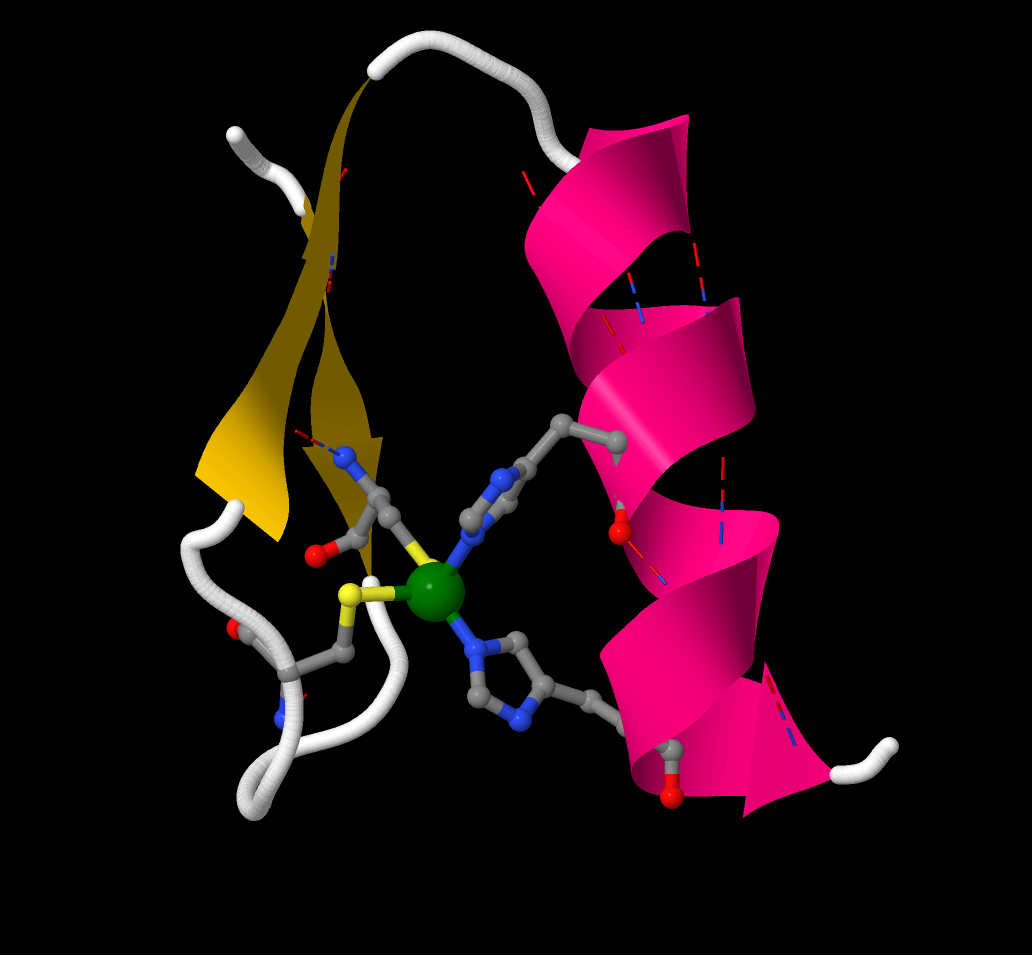
[Zn finger 1] shows a single zinc finger unit cut from a sequence of similar fingers. it comprises one short α-helix and two short sections of β-sheet
arranged around a Zn2+ ion (green).
[Zn finger 2] shows the coordination sphere of the zinc ion, the metal is
4-coordinate and its coordination geometry is approximately tetrahedral.
The ligands are two Cys from the β-sheet section (Cys7 and Cys12)
two His from the α-helical section (His25 and His29). So the sequence is:
... -Cys7-X9-X10-X11-Cys12-(X)14-His25-X26-X27-X28-His29- ...
The "finger" is a consequence of Zn coordination to these sites and the relatively rigid protein secondary
structure.
What would happen if the spacing between the two Cys groups (or two His groups) was different
- i.e. if there were a greater or lesser number of amino acids between them?
The next figures show a chain of three zinc fingers of a (mouse) transcription factor protein bound to a section of DNA.
In [Zn finger/DNA 1] both molecules are shown in cartoon form;
in [Zn finger/DNA 2] the DNA is shown in spacefilling mode,
and in the [Zn finger/DNA 3] both are shown as spacefilling views.
Note how the zinc finger protein fits into the major groove of the DNA.
Return to the index page.
Data from: Zinc Finger-DNA Recognition: Crystal Structure of a ZiF268-DNA complex at 2.1 Angstroms
NP Pavletich and CO Pabo, Science 252, 809, 1991 (PDB code 1ZAA).
Return to the index page.