Catalase & Peroxidase
This page shows some interactive JSmol views of catalase and peroxidase.
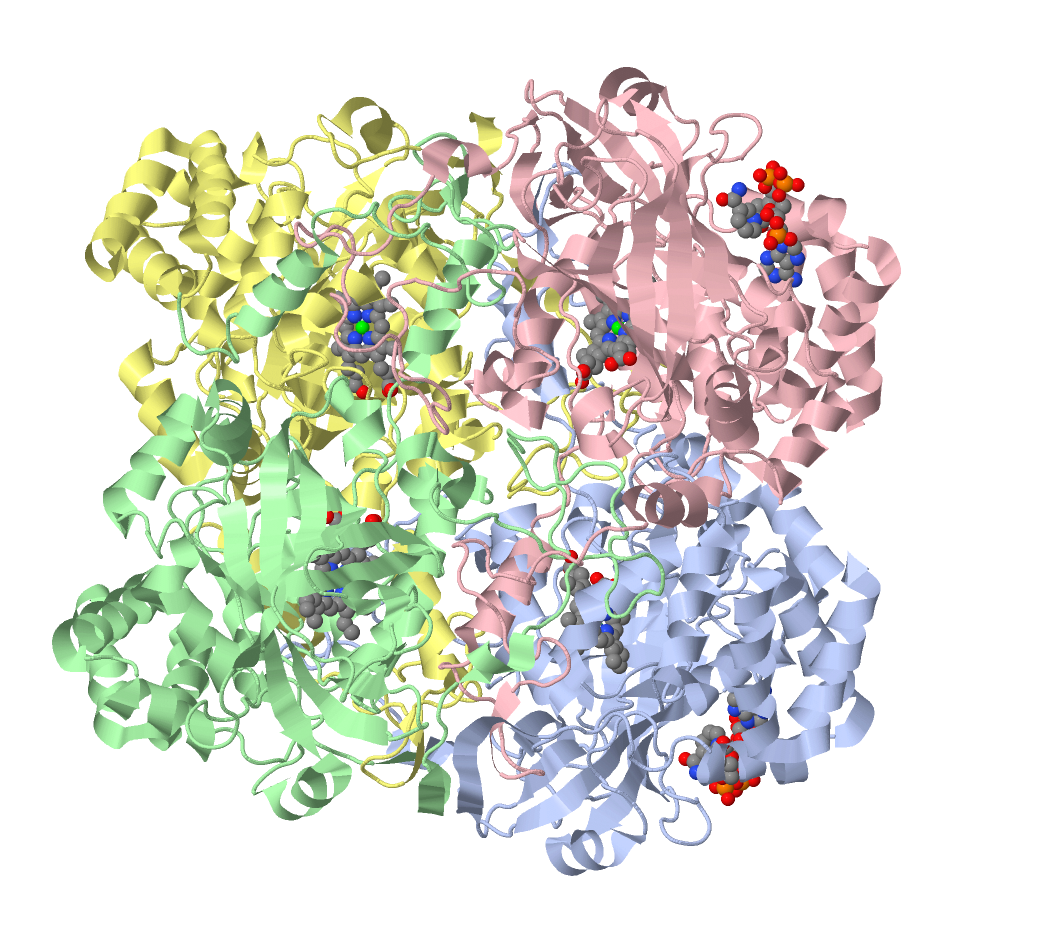
[Catalase 1] shows the tetrameric structure of (human) ctalase.
Each subunit conains one heme group,
the protein also contains some (bound) NADPH molecules, probably for receipt/delivery of reducing equivalents (H+).
[Catalase 2] shows the reduced (FeIII) resting state - which is 5-coordinate.
Note the Tyr residue coordinated to the iron, the water molecule and conserved distal His above the active site,
and the H-bonds linking them.
[Catalase 3] shows "Compound I of catalase", containing a ferryl-oxo (FeIV=O) group.
This is characterised by a very short Fe-O distance (here 1.76 Å) and by high reactivity.
Peroxidases are generally momomeric, again with one heme group per unit. This set of figures shows a ferrous form (photoreduced by X-rays) and the corresponding Compound I and Compound II - all for Cytochrome c peroxidase. The Fe-O distances are given below, Compound I is clearly shorter than Compound II, and it is suggested that Compound II contains FeIV-OH (ferryl hydroxide), with a single Fe-O bond.
- Reduced Cyt. c peroxidase Fe - O: 2.02 Å
- Compound I of Cyt c peroxidase Fe - O: 1.63 Å
- Compound II of Cyt c peroxidase Fe - O: 1.83 Å
Catalase (Compound I) data from: Structural studies of Proteus mirabilis catalase in its ground state, oxidized state and in complex with formic acid. Andreoletti, P., Pernoud, A., Sainz, G., Gouet, P., Jouve, H.M. (2003) Acta Crystallogr.,Sect.D 59: 2163-2168 (PDB code 1MQF).
Cytochrome c Peroxidase data from: Nature of the Ferryl Heme in Compounds I and II. Gumiero, A., Metcalfe, C., Pearson, A., Raven, E.L., Moody, P.C.E. (2011) J.Biol.Chem. 286: 1260-1268 (PDB 2XJ8, 2XIL and 2XJ5 )
Return to the index page.