Insulin.
This page shows some interactive JSmol views of the (human) insulin hexamer structure.
Explore the structures for yourself:
The first figure shows an overview of the structure. Note the large number of associated water molecules (red spheres).
The assembly contains 12 protein chains - 6 A subunits and 6 B subunits.
In the next view, the water molecules have been removed; the disulfide bonds and histidine residues are included, and each subunit
is colored differently.
The disulfide bonds (yellow) link cysteine amino acids on A and B subunits - giving 6 AB pairs (hence the "hexameric" structure)
Note that most of the histidine residues are involved in coordination to zinc - and these interactions play a major part in
holding the hexamer together.
(What other interactions contribute?)
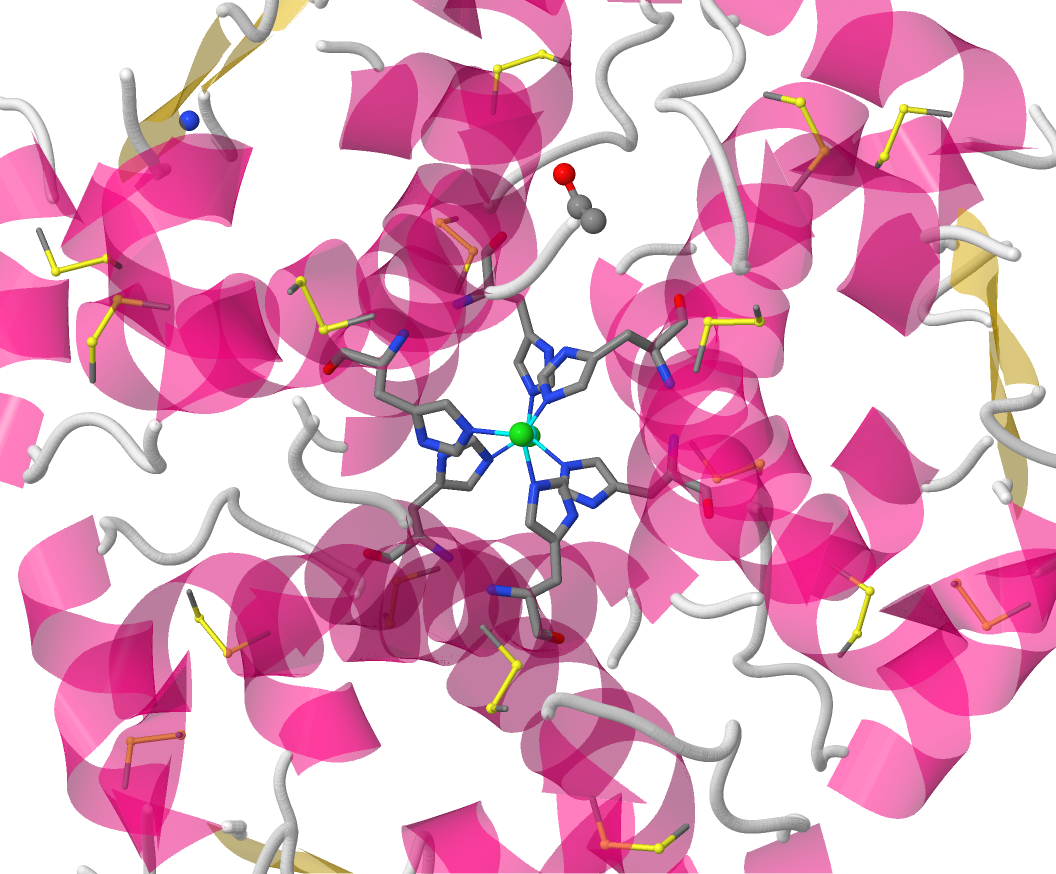
The final view emphasises the coordination sphere of the Zn2+ ion. The geometry at the metal ion is approximately tetrahedral and the
ligands are one chloride ion and three His residues, each from a different protein chain.
Return to the index page.
Data are taken from:
Insulin, monoclinic form (homo sapiens). to be published
Turkenburg, M.G.W., Whittingham, J.L., Turkenburg, J.P., Dodson, G.G., Derewenda, U., Smith, G.D., Dodson, E.J., Derewenda, Z.S., Xiao,
B. (PDB code 1ZNJ).
Return to the index page.