These are some of our structures selected because I think they are pretty cool (some may also be useful).
Click on the pictures for the 3D interactive versions, they may take a few seconds to load.
AgI(pmtbH)]4
Click image to activate Jmol
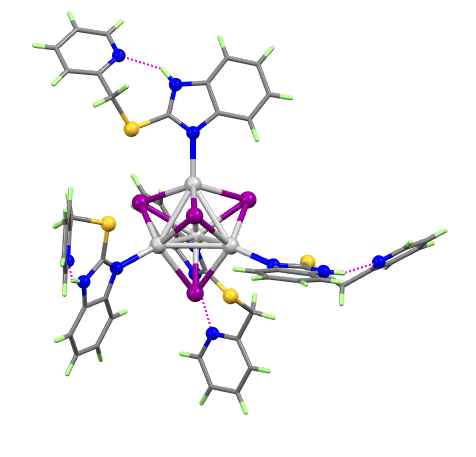
The tetrasilver complex [AgI(pmtbH)]4 (where pmtbH is 2-[(2-pyridinylmethyl)thio]-1H-benzimidazole) forms as a surprisingly compact cluster in which:
- four Ag(1) ions (silver) form a central Ag4 tetrahedron
- four iodide ions (purple), each cap a face of the tetrahedron
- four pmtbH ligands complete the coordination of the silver ions
- there are four weak S.....I interactions
- intramolecular NH.....N H-bonds satisfy the remaining bonding capacity
- the molecule has 4 point symmetry (it lies on a four-fold rotation-inversion axis)
A related polymeric complex, {[Ag4(pmtbH)4(NO3)4·2X}n (X = solvent), shows antimicrobial activity in vitro; and stimulates the immune system
of Galleria mellonella larvae in vivo. Preliminary mechanistic studies using flow cytometry suggest the antimicrobial response is linked
to bacterial programmed cell death. The Ag4 cluster is not biologically active but it is a much prettier structure.
Tetrameric and Polymeric Silver Complexes of the Omeprazole Scaffold; Synthesis,
Structure, in vitro and in vivo Antimicrobial Activities and DNA Interaction.
V. Jakobsen, L. Viganor, A. Blanco-Fernández, O. Howe, M. Devereux, C. J. McKenzie, and V. McKee.
J. Inorg. Biochem., 2018, 168, 317-328.
[Mg2Ti4(O)2(OH)4(CF3COO)8(THF)6]
Click image to activate Jmol
The cluster Mg2Ti4(O)2(OH)4(CF3COO)8(THF)6] is an example of a
set of bimetallic complexes, all with challenging crystallographic disorder problems. I like them because I invested a lot of time in them.
The structure was refined in space group Pbca with a disorder about the centre of symmetry which was not reduced by reducing the
symmetry of the system to Pca21 or P212121.
The core of the molecule consists of a tetrahedron of Ti(II) ions. Each edge of the tetrahedron is bridged by an oxygen atom,
generating a Ti4O6 adamantane cage. Four of the bridging species are hydroxo ions; the remaining two are
oxo ions which are also coordinated to Mg(II) ions.
The disorder arises from Ti ions occupying alternative sites (by inversion or 2-fold rotation), generating an overlapping adamantine
with the oxygen atoms in the same positions. Four trifluoroacetate ions are bonded to each magnesium ion, each bridging to a
titanium ion. All of the metal ions are six-coordinate, the last binding site being filled by one THF ligand for each metal ion.
Rather sadly, these neat molecules are designed and synthesised in order to be decomposed easily to form mixed-metal oxide layers for
photoelectrochemical applications.
MgTi2O5, Thin Films from Single Molecular
Precursor for Photoelectrochemical Water Splitting.
M. A. Ehsan, R. Naeem, V. McKee, A. H. Saeed, and M. Mazhar.
Solar Energy Materials and Solar Cells, 2017, 161, 328-337.
This one is only a movie, it is not interactive.
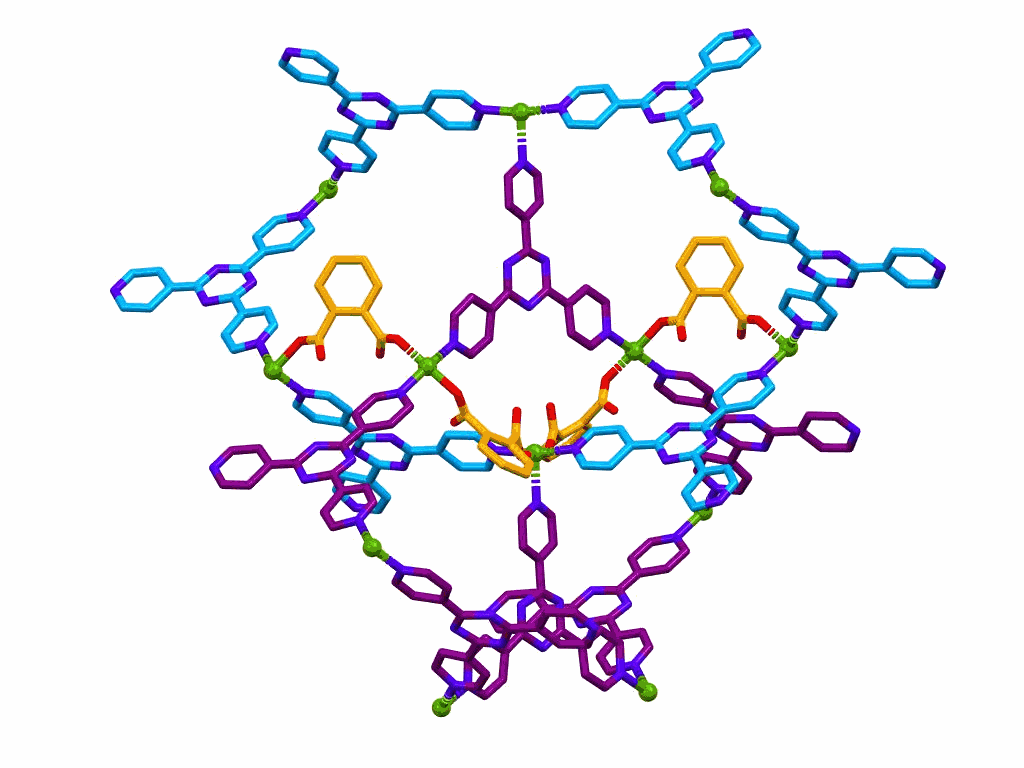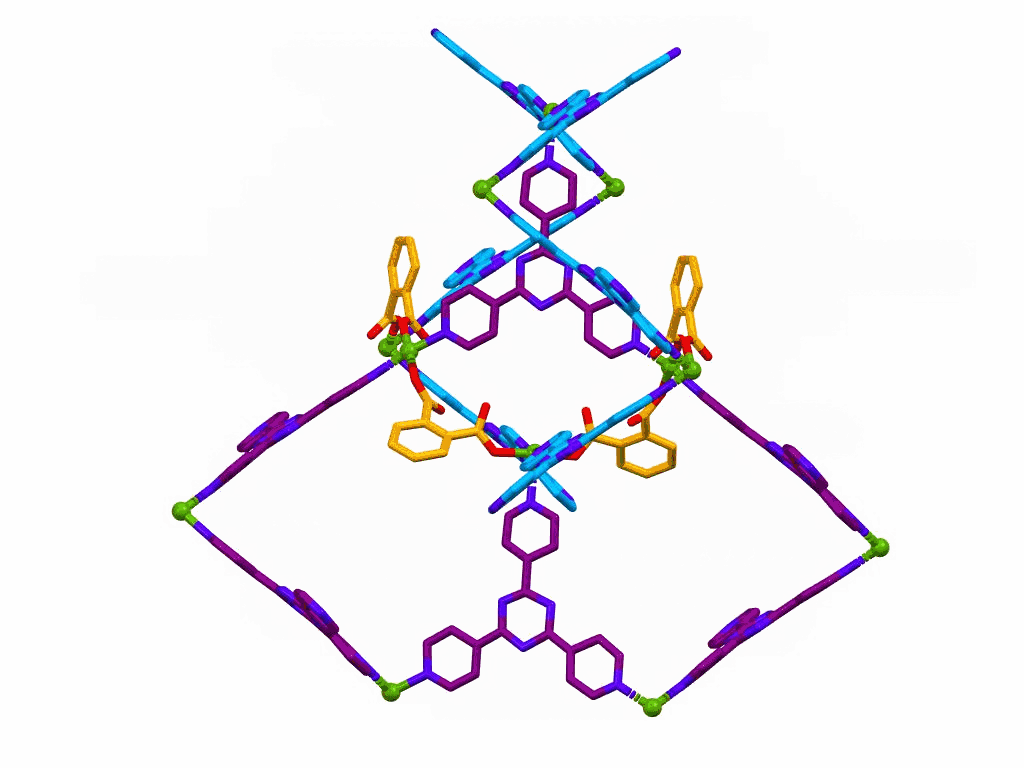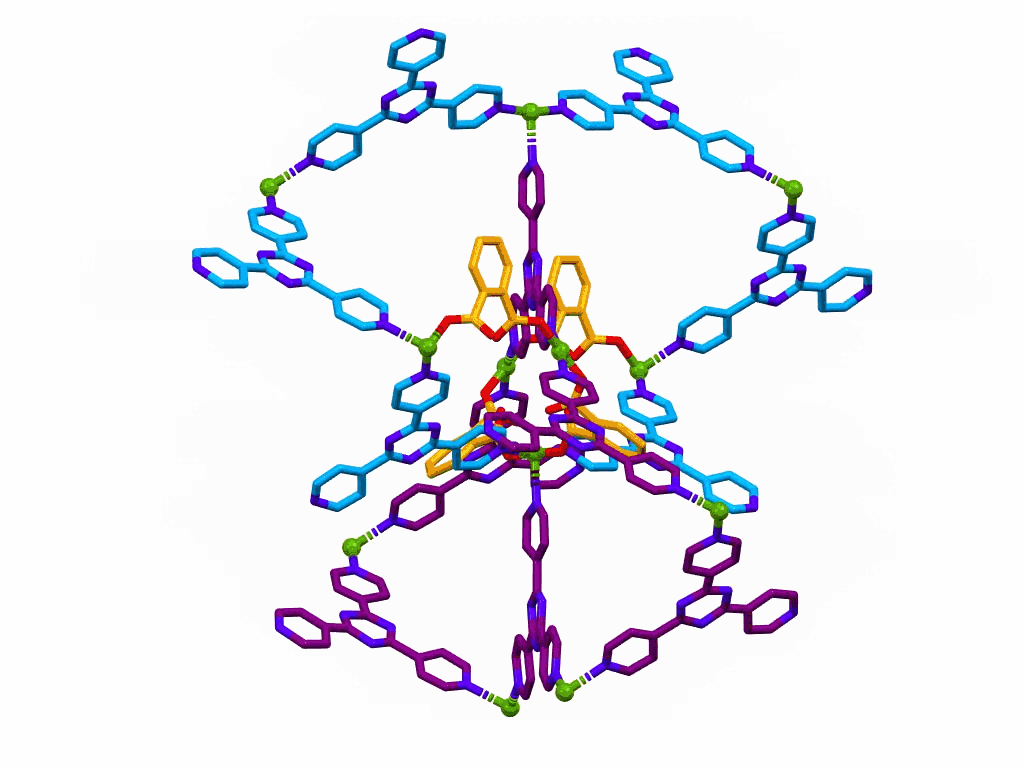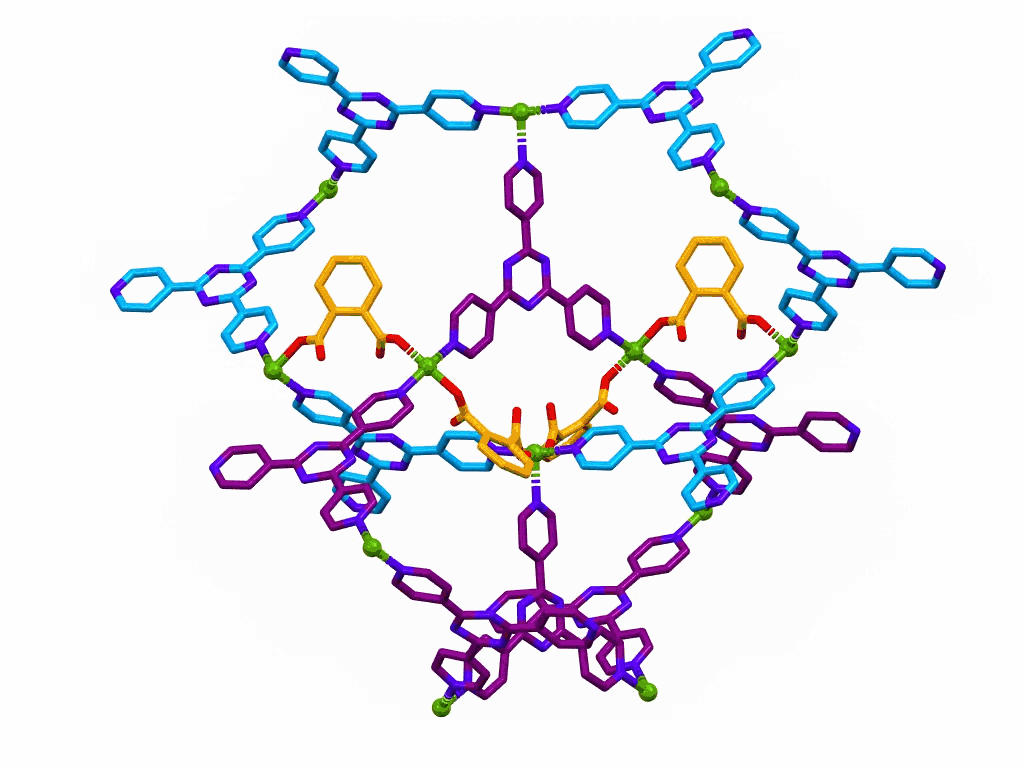
The movie shows a section of a MOF prepared from:
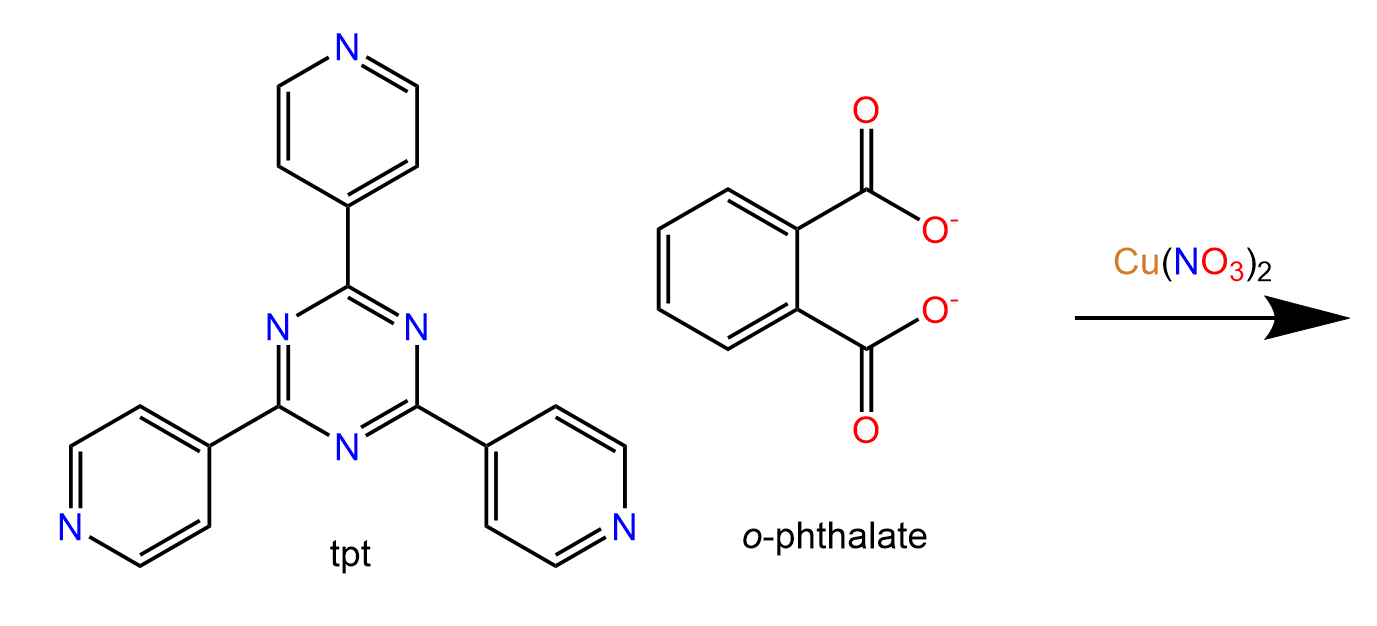
This structure was published in a special issue of Aust. J. Chem. in honour of Prof Richard Robson,
a pioneer of MOF chemistry.
Reversible and Vapochromic Chemisorption of Ammonia by a Copper(II) Coordination Polymer.
C. Wegeberg, D. Nielsen, S. Mossin, B. F. Abrahams, V. McKee and C.J. McKenzie.
Aust. J. Chem., 2019, 72, 817-826.